
保持疫苗效力

每年,疫苗可预防 200 万至 300 万人死亡。根据世界卫生组织的数据,由于在 2000 年至 2016 年期间接种了疫苗,全世界与麻疹相关的死亡人数减少了 84%。在过去 10 年中,超过 10 亿儿童接种了疫苗。妥善处理和储存是保持疫苗有效性的必要条件。
在特定准则下处理疫苗的理由可以从了解其成分中找到。疫苗是生物制品,含有蛋白质,通常还含有活病毒(但已被削弱)。疫苗通常包含以下成分:1) 抗原:活性成分,无论是活的(但已被削弱)还是灭活的,都会引发免疫反应。疫苗的作用是刺激人的免疫系统产生对特定疾病的免疫力。2) 铝凝胶或铝盐:佐剂可促进对疫苗产生更好、更持久的反应。佐剂可最大限度地减少所需的抗原量。3) 抗生素:用于某些疫苗中,以防止细菌在生产和储存过程中滋生。4) 鸡蛋蛋白:存在于用鸡蛋制成的疫苗中,如流感和黄热病疫苗。5) 谷氨酸钠(味精)和 2-苯氧基乙醇:用于某些疫苗,以防止热、光、酸度或湿度的负面影响。6) 硫柳汞:一种含汞的防腐剂,用于防止污染和有害细菌。不过,大多数疫苗已不再含有这种成分。7) 悬浮液:无菌水、生理盐水或含有蛋白质的液体。疫苗中还含有甲醛,用于杀死生产过程中可能污染疫苗的病毒和细菌。大部分甲醛会在包装前去除。
有些疫苗必须冷藏,有些则需要冷冻。了解每种疫苗的具体要求对于保持效力、避免浪费产品和金钱损失至关重要。有时在接种疫苗后才发现疫苗不再有效。2017 年,加利福尼亚州文图拉县的卫生官员意识到,疫苗可能在接种前被意外冷冻。不幸的是,没有办法确定谁接种了坏疫苗,因此,23000 人收到了警报,并可以选择重新接种。如果第二次为所有 23000 名患者接种疫苗,该县将额外花费 130 万美元。市民不满的影响可能会导致更高的价格。
在疫苗获准使用之前,需要对其进行大量测试,以确保疫苗对消费者有效且安全。对于大多数疫苗而言,这一评估期长达数年。在流行病和大流行期间,这一测试期可能会缩短。冠状病毒就是一个例子。COVID-19 疫苗在几个月内就被确定,而不是几年。既然我们已经有了可行的疫苗,妥善处理就势在必行。
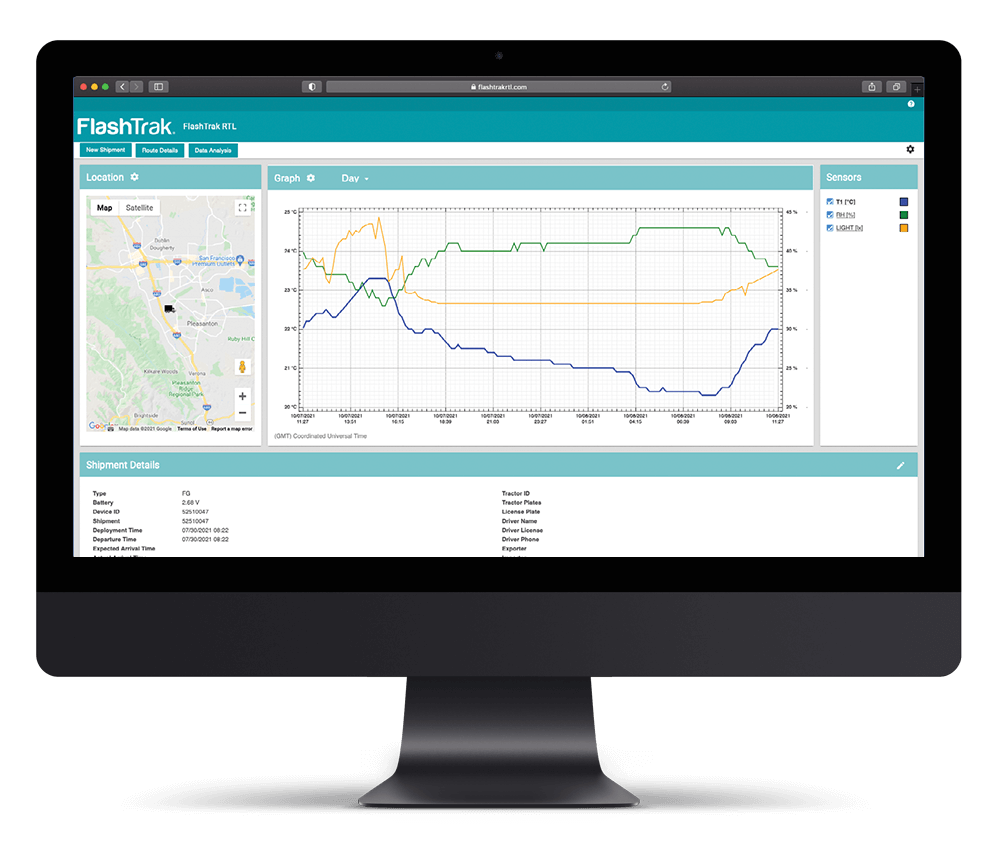
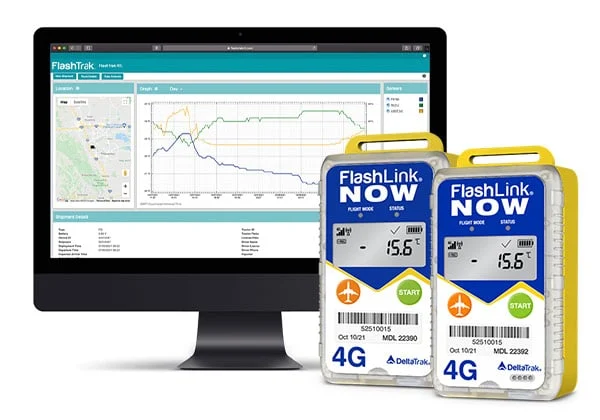
疫苗冷链是一个由各种要求、专用设备和程序组成的重要系统,从疫苗生产到接种都要进行维护。要使疫苗冷链有效,三个要素至关重要:训练有素的员工、准确的库存管理以及可靠的储存和温度监控。冷链非常重要,任何温度偏差都会对疫苗的有效性产生负面影响。例如,如果疫苗不是用来冷冻的,那么只要温度降至 32 华氏度或以下,疫苗就会完全失效。更糟糕的是,通常不会有任何直观的迹象表明疫苗已经受损。疫苗的救生能力非常宝贵,我们不能冒这个险。使用的温度监控设备应适合疫苗,并配备警报器,以提醒利益相关者注意温度偏差。
在当地运输疫苗通常是为了在医疗服务提供者之间共享、应对紧急情况(应制定 SOP)、转移到异地诊所或快速使用有过期危险的库存。不过,这些都是特殊情况。疫苗不应常规运输。在必须运输时,应始终使用适当的便携式运输装置、包装材料和温度监控装置。如果在新地点收到疫苗后没有储存设备,建议在运输过程中使用便携式疫苗冰箱。如果没有便携式冰箱,也可以使用以下方法:合格的容器包装;有条件的水瓶运输系统;制造商的原始运输容器(仅在没有其他选择的情况下)。上述每种方法都应配备温度监控装置。
在紧急情况下,应采取某些特定措施来保存疫苗库存:
- 联系替代储存设施,为运输疫苗做好准备;
- 只有在准备包装或恢复供电时才打开冰箱;
- 只要预计会发生紧急情况,如恶劣天气,就暂停接种疫苗,为运输做好准备。
长距离运输疫苗(如冠状病毒疫苗)需要考虑一些特殊因素。例如,最近批准的辉瑞公司的 COVID-19 疫苗必须在 -70°C 下储存和运输。为了保持适当的温度,公司使用了可容纳 20 磅干冰颗粒的特殊运输容器。医疗服务提供者收到疫苗并配发后,对储存有特殊要求,如经常向储存容器中添加干冰颗粒,并限制打开容器的次数。Moderna 和 Astra-Zeneca 公司生产的疫苗需要分别在 20°C 和 2-8°C条件下运输和储存。
目前,冠状病毒疫苗的分发工作已全面展开,2020 年 10 月成立的美国联邦航空局COVID-19 疫苗空运小组将应对任何与空运有关的问题,并确保每天 24 小时不间断运输。在管理 COVID-19 疫苗冷链的过程中,将需要专门设计用于各种温度曲线的温度监测设备。鉴于 COVID-19 疫苗的分发速度很快,实时监控使利益相关者能够在发现温度偏差时采取果断措施。在快节奏的环境中经常会发生忘记激活数据记录器的情况,但有了影子日志等专利功能,这种情况就不再是世界末日了。DeltaTrak 提供多种解决方案来满足您的疫苗运输和储存需求。如需了解更多信息,请联系 DeltaTrak 专业销售人员。
参考文献
世界卫生组织,https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage,2020 年 7 月 15 日。
世界卫生组织,https://www.who.int/campaigns/immunization-week/2018/en/, 2018。
世界卫生组织/免疫联盟。模块 2:疫苗和药物:相似之处和不同之处》, https://isoponline.org/wp-content/uploads/2015/10/Differences-on-drugs-and-vaccines.pdf,2015 年。
Heredia Rodriguez, C. “Vaccines are Sometimes Stored Improperly, Reducing Their Effectiveness”, USAtoday.com,https://www.usatoday.com/story/news/health/2019/02/11/cdc-vaccines-children-stored-improperly-mishandled-reduce-effectiveness-dhhs-measles/2811629002/, February 12, 2019.
Weise, E and Aspegran, E. “FAA Confirms First ‘Mass Air Shipment’ of Pfizer’s COVID-19 Vaccine from Belgium As US Preps for Distribution,” https://www.usatoday.com/story/news/health/2020/11/29/pfizer-covid-vaccine-faa-belgium-air-shipment/6445339002/, November 29, 2020.